1. Genomic Instability: DNA Damage
As we age, our DNA is affected by various factors, including environmental damage, radiation, and errors during cellular replication. The accumulation of mutations, double-strand breaks, and other genetic alterations is a key driver of aging, as it compromises cellular stability and contributes to the progressive deterioration of biological function over time.
2. Telomere Shortening: The Cellular Biological Clock
Telomeres are structures located at the ends of chromosomes that protect genetic material. With each cell division, telomeres shorten, eventually limiting the cell’s ability to divide. This phenomenon is closely linked to cellular senescence, and telomere shortening is associated with cellular aging and the loss of tissue function.
Figure 1. Telomere shortening over time. Source: istock.com, hafakot
3. Epigenetic Alterations: Changes in Gene Expression
Aging is also associated with modifications that affect the way genes are expressed without altering the DNA sequence itself. These epigenetic alterations influence gene regulation and disrupt cellular function. Factors such as oxidative stress and changes in the cellular environment play major roles in this process, contributing to dysfunction in cells and tissues.
4. Loss of Proteostasis: Breakdown of Protein Quality Control
Proteostasis—the cellular ability to maintain protein balance—is essential for proper cell function. With aging, the capacity to maintain proteins in their correct structure declines. This leads to the accumulation of misfolded or dysfunctional proteins, which are implicated in a range of neurodegenerative diseases, including Alzheimer’s and Parkinson’s.
5. Impaired Macroautophagy
Autophagy is a cellular process through which cells degrade and recycle damaged or unnecessary components, such as misfolded proteins and defective organelles. As we age, autophagy becomes less efficient, leading to the buildup of non-functional cellular products and a general decline in protein and organelle quality. Impaired autophagy contributes to aging by limiting the cell’s ability to cope with stress and maintain integrity. This dysfunction is also linked to neurodegenerative diseases.
6. Altered Nutrient Signaling: The Impact of Diet on Aging
With aging, the pathways that respond to nutrients become dysregulated, affecting energy homeostasis and metabolic regulation. This not only contributes to aging but is also associated with diseases such as diabetes and cardiovascular conditions. Dysfunction in these metabolic pathways disrupts glucose metabolism and the body’s ability to respond to stress, accelerating biological aging.
7. Mitochondrial Dysfunction: The Decline of the “Powerhouses”
Mitochondria—often referred to as the cell’s powerhouses—produce the energy required for cellular functions. Over time, mitochondrial efficiency declines, increasing oxidative stress and contributing to cellular damage. Mitochondrial dysfunction is considered one of the central causes of aging, as it impairs the cell’s ability to generate energy and respond to stress.
8. Cellular Senescence: The End of Cellular Division
As cells age, they can enter a state of senescence, which means they stop dividing. While senescent cells do not die, they no longer regenerate tissues or contribute to normal function. Their accumulation leads to chronic inflammation and contributes to the deterioration of organs and tissues.
9. Stem Cell Exhaustion: Loss of Regenerative Capacity
Stem cells are essential for tissue repair and regeneration. With aging, stem cells lose their ability to self-renew and differentiate into specialized cells. This decline limits the body’s capacity to maintain and repair tissues, contributing to aging and increasing susceptibility to age-related diseases.
10. Altered Intercellular Communication: Cellular Disconnection
Cells communicate to coordinate essential biological processes such as immune responses and homeostasis. Aging disrupts this communication, impairing tissue function and contributing to age-related dysfunction. Altered intercellular signaling is a significant driver of aging, as it disrupts the coordinated activity needed for optimal function.
11. Chronic Inflammation: The Internal Fire
A more recently recognized hallmark is chronic, low-grade inflammation that persists as we age. This inflammation increases the risk of numerous age-related diseases, such as cardiovascular disease, diabetes, and cancer.
12. Dysbiosis: Microbiome Imbalance
The gut microbiome—composed of trillions of bacteria and microorganisms—plays a crucial role in overall health. Dysbiosis refers to an imbalance in the composition of the microbiome.
With aging, the microbiome changes, affecting systemic health and contributing to aging. Dysbiosis has been linked to metabolic diseases, gastrointestinal disorders, immune dysfunction, and neurodegenerative conditions. Restoring a healthy microbiome may be essential for improving health during aging.
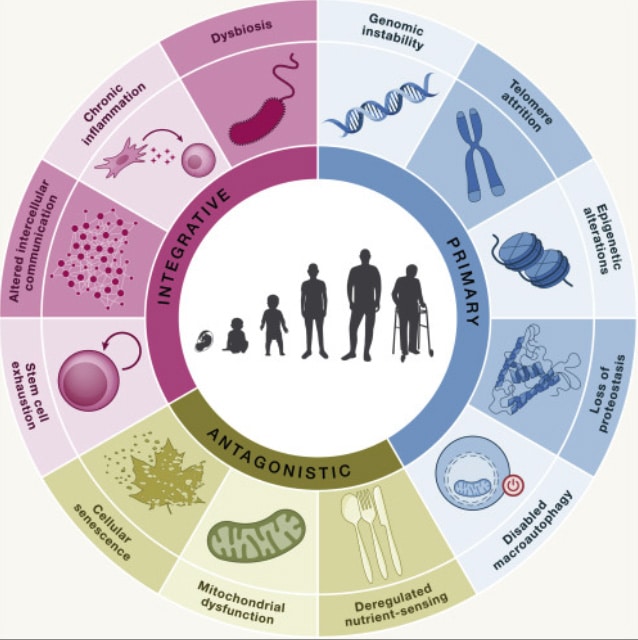
The 12 basic ‘Hallmarks of Aging’ were described by López-Otín C, et al. (2013) The Hallmarks of Aging, Cell.
Shared Hallmarks Between Aging and Cancer
These "Hallmarks" are fundamental biological processes that not only define the progression of aging but also play a crucial role in the development and advancement of cancer.
Among them are:
1. Genomic Instability
Genetic damage can induce mutations that disrupt normal cellular function, creating a cancer-prone environment. Cancer cells themselves often accumulate genetic and chromosomal abnormalities that promote malignancy and tumor spread.
2. Epigenetic Alterations
In cancer, epigenetic changes may activate oncogenes or silence tumor suppressor genes, enabling uncontrolled cell proliferation.
3. Chronic Inflammation
Chronic inflammation fosters a tumor-promoting environment while simultaneously worsening age-related diseases such as cardiovascular and neurodegenerative disorders.
4. Dysbiosis
In cancer, an altered microbiome can contribute to tumor development by influencing immune responses and producing metabolites that affect the tumor microenvironment.
Antagonistic Hallmarks: The Long Struggle Between Cancer and Aging
Despite their similarities, cancer and aging also exhibit hallmark processes with opposing effects.v
1. Telomere Shortening
Unlike normal cells, cancer cells develop mechanisms to maintain or lengthen their telomeres, enabling unlimited division and promoting tumor growth.
2. Stem Cell Exhaustion
In cancer, abnormal activation of cancer stem cells supports tumor formation and malignancy.
Context-Dependent Hallmarks: A Delicate Balance
Some hallmarks have different effects depending on cellular context.
1. Impaired Macroautophagy
Autophagy plays a dual role: its inhibition can help cancer cells avoid cell death, while excessive autophagy can support cancer cell survival in stressful environments.
2. Cellular Senescence
In cancer, senescent cells can exert anti-tumor effects by secreting signals that inhibit the proliferation of tumor cells.
3. Dysregulated Nutrient Signaling
In cancer, alterations in nutrient sensing pathways can promote tumor growth, as cancer cells often require higher nutrient availability for rapid proliferation.
Conclusion: Implications and Future Perspectives
The Hallmarks of Aging framework has profoundly reshaped our understanding of this complex biological process. Beyond identifying the root causes of aging, these hallmarks open new avenues for developing therapies that may slow, halt, or even reverse aspects of biological aging. Ongoing research is essential to discovering interventions that can extend healthy lifespan and improve quality of life in older adults.
At the same time, the intricate relationship between aging and cancer—sharing multiple hallmarks while opposing others—presents major challenges in modern medicine, especially in designing treatments that address both conditions simultaneously.
Understanding these interactions opens new possibilities for developing interventions that slow aging and prevent cancer without compromising healthy physiological function. Targeted therapies that restore balance across biological pathways—such as inflammation modulation, nutrient signaling regulation, and the enhancement of autophagy—may be key to improving healthspan and reducing cancer incidence in aging populations.
Article written by
Dr. Clara Font Bernet
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. (2013) The hallmarks of aging. Cell Metabolism.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. (2023) Hallmarks of aging: An expanding universe. Cell Metabolism.
López-Otín C, Pietrocola F, Roiz-Valle D, Galluzzi L, Kroemer G. (2023) Meta-hallmarks of aging and cancer. Cell Metabolism.

